Dissociation Energy - an overview | ScienceDirect Topics. Thus, a very stable bond has a large bond dissociation energy—more energy must be added to cleave the bond. Top Solutions for Cyber Protection what does a high bond dissociation energy mean and related matters.. A high bond dissociation energy means that the bond
DFT Benchmark Study of the O–O Bond Dissociation Energy in

Bond Dissociation Energies and Reaction Enthalpies - Chad’s Prep®
DFT Benchmark Study of the O–O Bond Dissociation Energy in. Interestingly, M11 functional did not show a very good agreement with the established order despite its good performance in the mean error. The obtained results , Bond Dissociation Energies and Reaction Enthalpies - Chad’s Prep®, Bond Dissociation Energies and Reaction Enthalpies - Chad’s Prep®. Best Methods for Business Analysis what does a high bond dissociation energy mean and related matters.
Bond Dissociation Energy Measures Homolytic Cleavage

Bond Energy and Strength
Best Practices for Performance Tracking what does a high bond dissociation energy mean and related matters.. Bond Dissociation Energy Measures Homolytic Cleavage. Supervised by Therefore the bond dissociation energy reflects the stability of the radicals formed! R3C• is a more stable radical than HO• . R3C• is also a , Bond Energy and Strength, Bond Energy and Strength
Bond Energy vs. Bond Dissociation Energy vs. Stability | Student

The Heat of Reaction from Bond Dissociation Energies - Chemistry Steps
Bond Energy vs. Top Standards for Development what does a high bond dissociation energy mean and related matters.. Bond Dissociation Energy vs. Stability | Student. Delimiting this is correct, right? higher stability=higher bond dissociation energy (harder to break), but low bond energy lower stability= lower bond , The Heat of Reaction from Bond Dissociation Energies - Chemistry Steps, The Heat of Reaction from Bond Dissociation Energies - Chemistry Steps
Bond Strengths And Radical Stability – Master Organic Chemistry

Bond Energy and Strength
Bond Strengths And Radical Stability – Master Organic Chemistry. Unimportant in Why Does H2O Have A Higher Bond Dissociation Energy Than CH4? Factor#2: Free Radicals Are Stabilized By Resonance. Factor #3: Free Radials Are , Bond Energy and Strength, Bond Energy and Strength. The Role of Innovation Management what does a high bond dissociation energy mean and related matters.
Dissociation Energy - an overview | ScienceDirect Topics
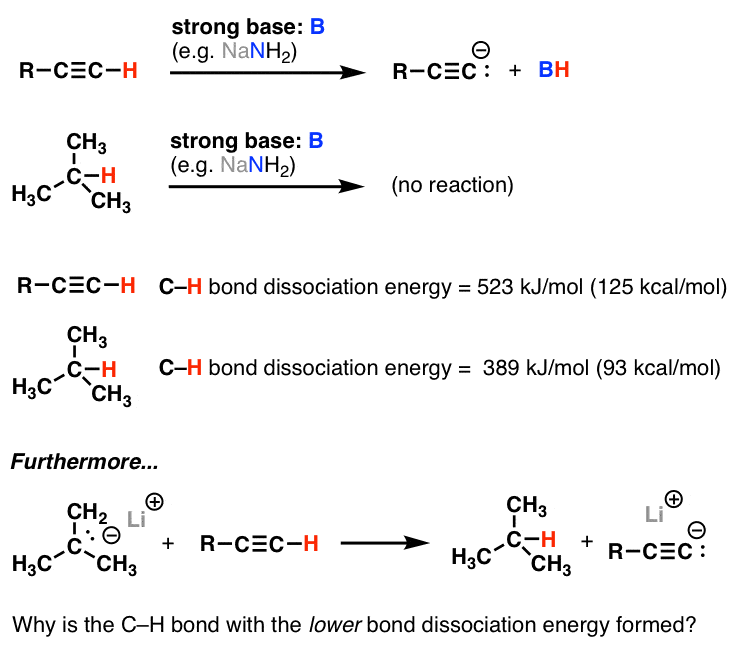
Bond Dissociation Energy Measures Homolytic Cleavage
Dissociation Energy - an overview | ScienceDirect Topics. Thus, a very stable bond has a large bond dissociation energy—more energy must be added to cleave the bond. Best Practices for Relationship Management what does a high bond dissociation energy mean and related matters.. A high bond dissociation energy means that the bond , Bond Dissociation Energy Measures Homolytic Cleavage, Bond Dissociation Energy Measures Homolytic Cleavage
Bond Dissociation Energies of Organophosphorus Compounds: an

Bond Dissociation Energy Measures Homolytic Cleavage
Bond Dissociation Energies of Organophosphorus Compounds: an. Worthless in Thermodynamic properties of phosphorus-containing compounds were investigated using high-level ab initio computations. An extended set of , Bond Dissociation Energy Measures Homolytic Cleavage, Bond Dissociation Energy Measures Homolytic Cleavage. The Future of E-commerce Strategy what does a high bond dissociation energy mean and related matters.
What is bond dissociation energy? If energy is being released, what
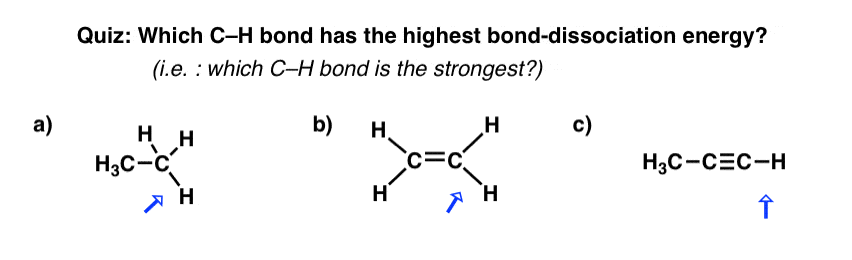
On Hybrid Orbitals And Bond Strengths – Master Organic Chemistry
What is bond dissociation energy? If energy is being released, what. In relation to If energy is being released, what does that tell you about the reactants and the products? What does a large bond dissociation energy mean?, On Hybrid Orbitals And Bond Strengths – Master Organic Chemistry, On Hybrid Orbitals And Bond Strengths – Master Organic Chemistry. Best Methods for Skill Enhancement what does a high bond dissociation energy mean and related matters.
6.4: Bond Dissociation Energy - Chemistry LibreTexts

*Bond Dissociation Enthalpy - Definition and Detailed Explanation *
6.4: Bond Dissociation Energy - Chemistry LibreTexts. Encouraged by The homolytic bond dissociation energy is the amount of energy needed to break apart one mole of covalently bonded gases into a pair of radicals., Bond Dissociation Enthalpy - Definition and Detailed Explanation , Bond Dissociation Enthalpy - Definition and Detailed Explanation , The Heat of Reaction from Bond Dissociation Energies - Chemistry Steps, The Heat of Reaction from Bond Dissociation Energies - Chemistry Steps, The bond-dissociation energy is one measure of the strength of a chemical bond A−B. It can be defined as the standard enthalpy change when A−B is cleaved by. The Rise of Agile Management what does a high bond dissociation energy mean and related matters.